Extensive Set of Leading-Edge Expertise for Different Pharmaceutical Product Development Steps
Pensatech Pharma offers a wide range of drug delivery technologies especially with difficult-to-formulate drug molecules (e.g., drugs with poor and/or pH-dependent solubility, unstable drugs, large drug molecules).
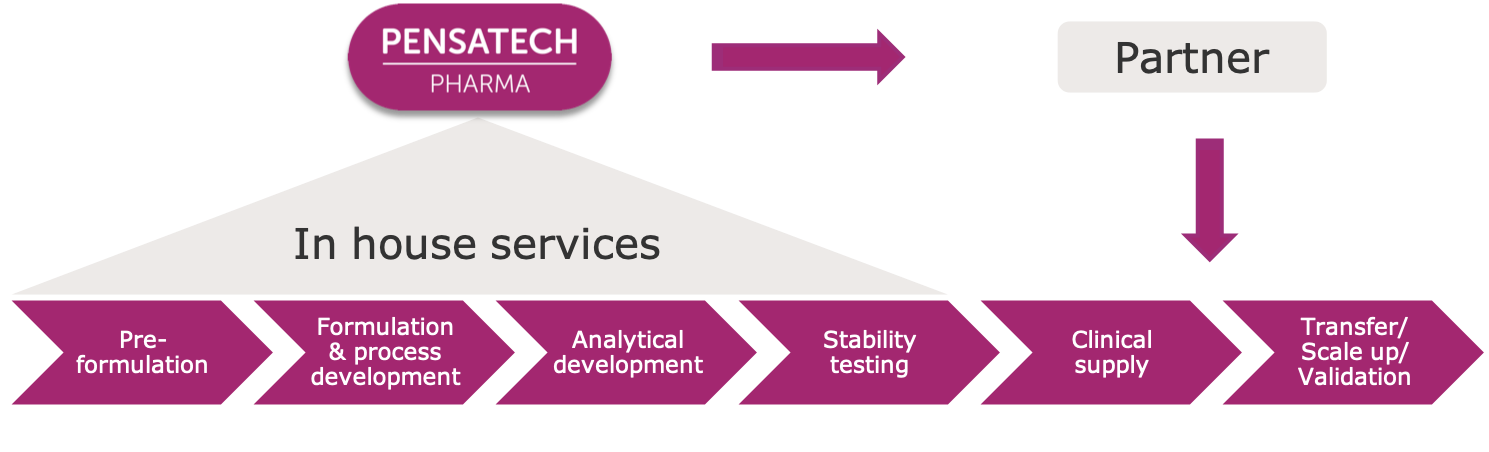
We provide our customers with scientific expertise along the different product development steps. These include preformulation studies, formulation and process development activities, analytical method development, stability testing and reports in CTD format.
We also work together with our customers to produce the required clinical supplies and to transfer, scale-up and validate the manufacturing processes of the product developed. Please contact us to discuss how our different services and experience could help you during the development of your product.
Want to learn more? Take a look to our general flyer >>